LTFU Study - File 1
LTFU Global were tasked with locating 353 lost-to-follow-up (LTFU) patients who had previously consented to participate in clinical trials. These patients had become unresponsive or unreachable during the course of the study, posing a significant challenge to data integrity and the overall success of the trials. The importance of reconnecting with these patients cannot be overstated, as their continued participation is crucial for ensuring accurate, comprehensive trial results. With our proven expertise in patient tracing and compliance, LTFU Global launched a focused effort to locate each individual, using advanced tracking methods to re-establish communication.
Our team at LTFU Global understands the complexities involved in tracing LTFU patients, especially when dealing with global clinical trials. We employed a combination of innovative technology and personalised outreach to ensure the highest chances of success. The process involved verifying patient contact details, performing vital status checks, and re-engaging patients who had lost touch with the clinical teams. Through our efforts, we aim to improve follow-up care and ensure that of the 334 patients were accounted for, ultimately enhancing the accuracy and outcomes of the clinical trials.
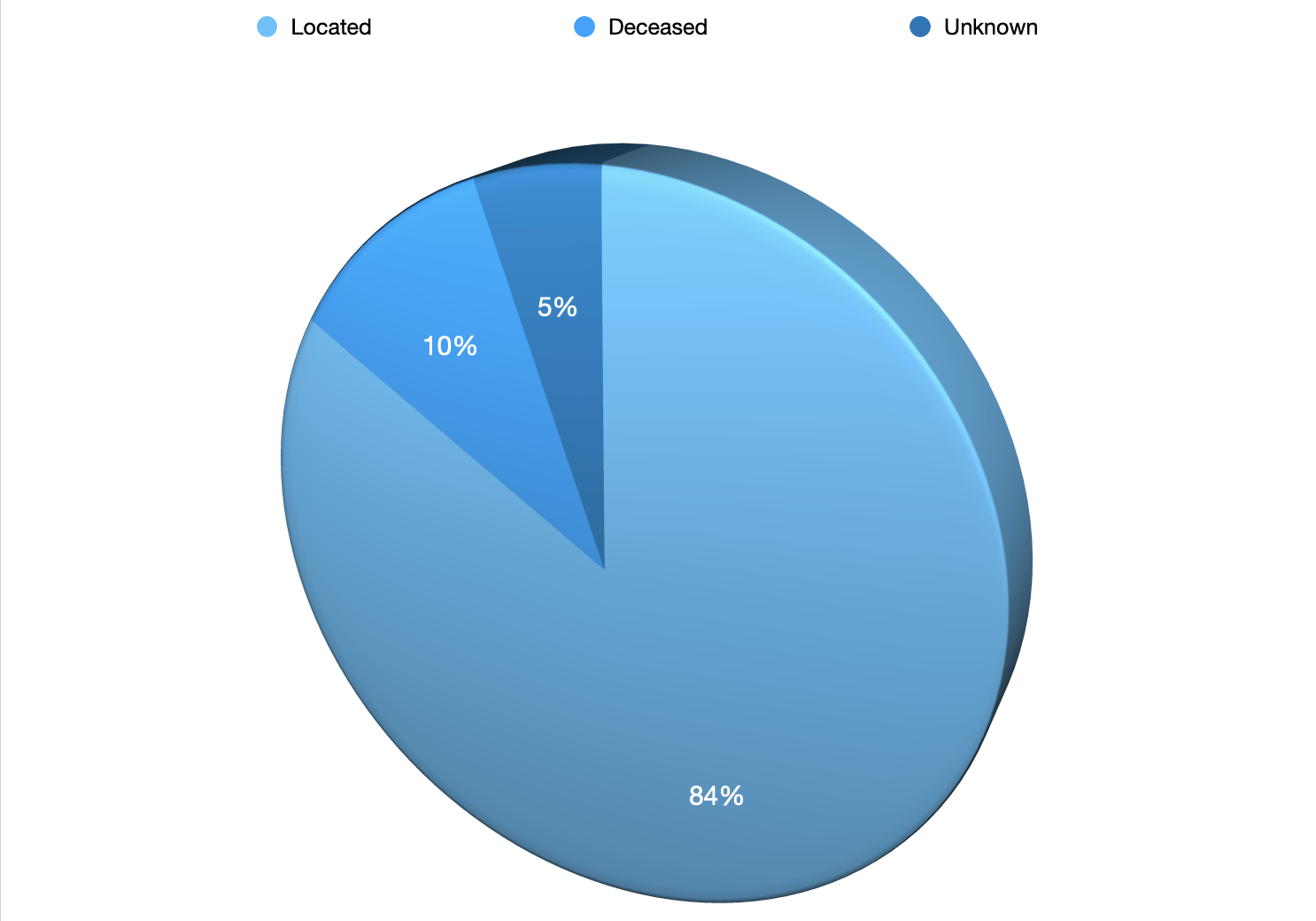
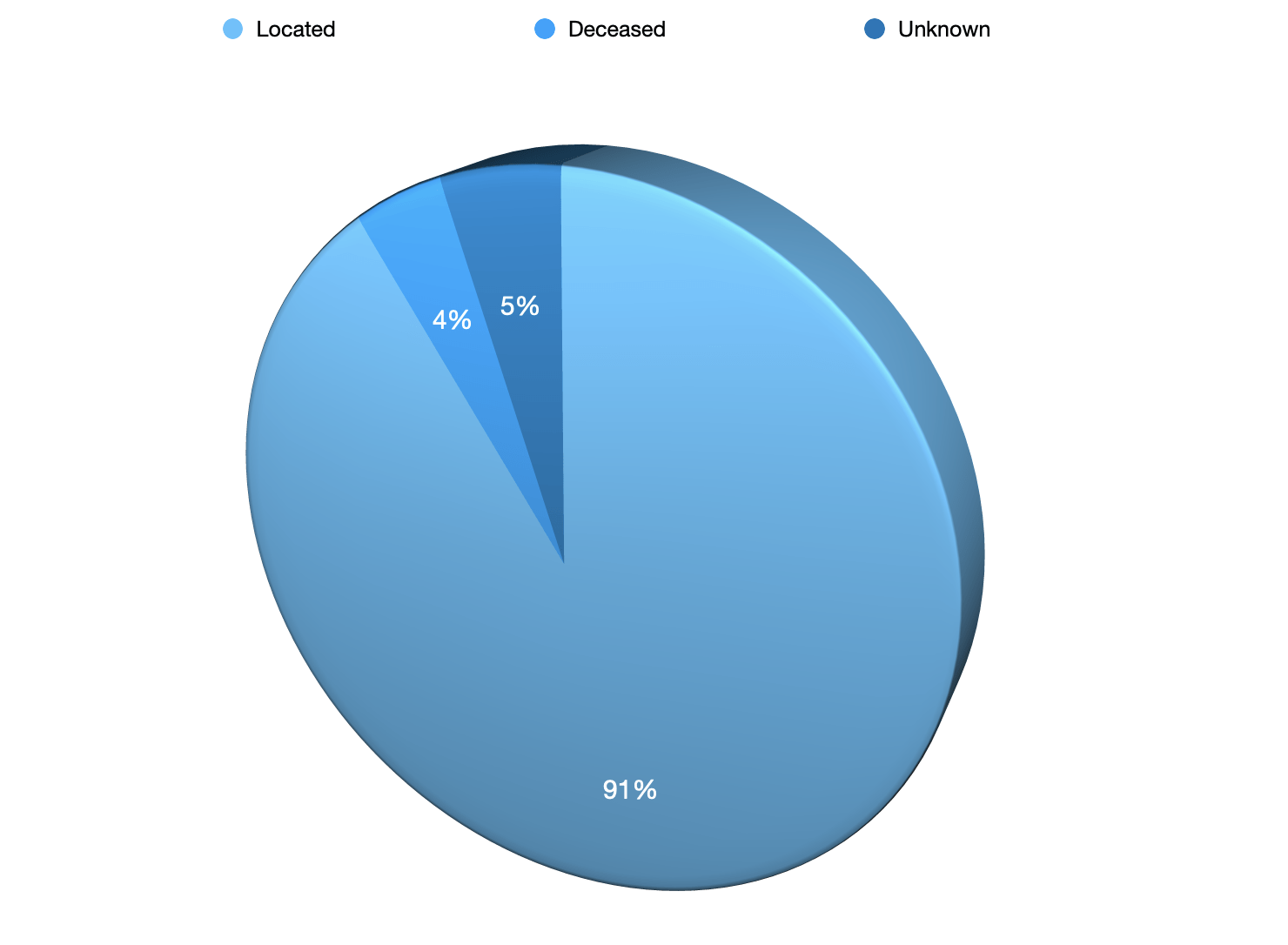
297 Located
37 Deceased
19 Unknown
LTFU Study - File 2
LTFU Global were assigned the critical responsibility of locating 98 lost-to-follow-up (LTFU) patients who had previously consented to participate in clinical trials. These patients had become unresponsive or unreachable during the study, creating a significant challenge for maintaining data integrity and achieving successful trial outcomes. Reconnecting with these individuals was essential, as their ongoing participation was key to ensuring accurate and comprehensive trial data. Drawing on our extensive experience in patient tracing and compliance, LTFU Global initiated a targeted campaign, utilising advanced tracing techniques to re-establish contact with the patients.
At LTFU Global, we fully understand the challenges associated with tracing LTFU patients, especially in the context of international clinical trials. To optimise results, we employed a strategic blend of cutting-edge technology and personalised outreach. This process included verifying patient contact information, conducting vital status checks, and re-engaging those who had lost contact with the clinical teams. Thanks to our efforts, we successfully accounted for 92 patients, significantly improving follow-up care and enhancing the overall accuracy and outcomes of the clinical trials.
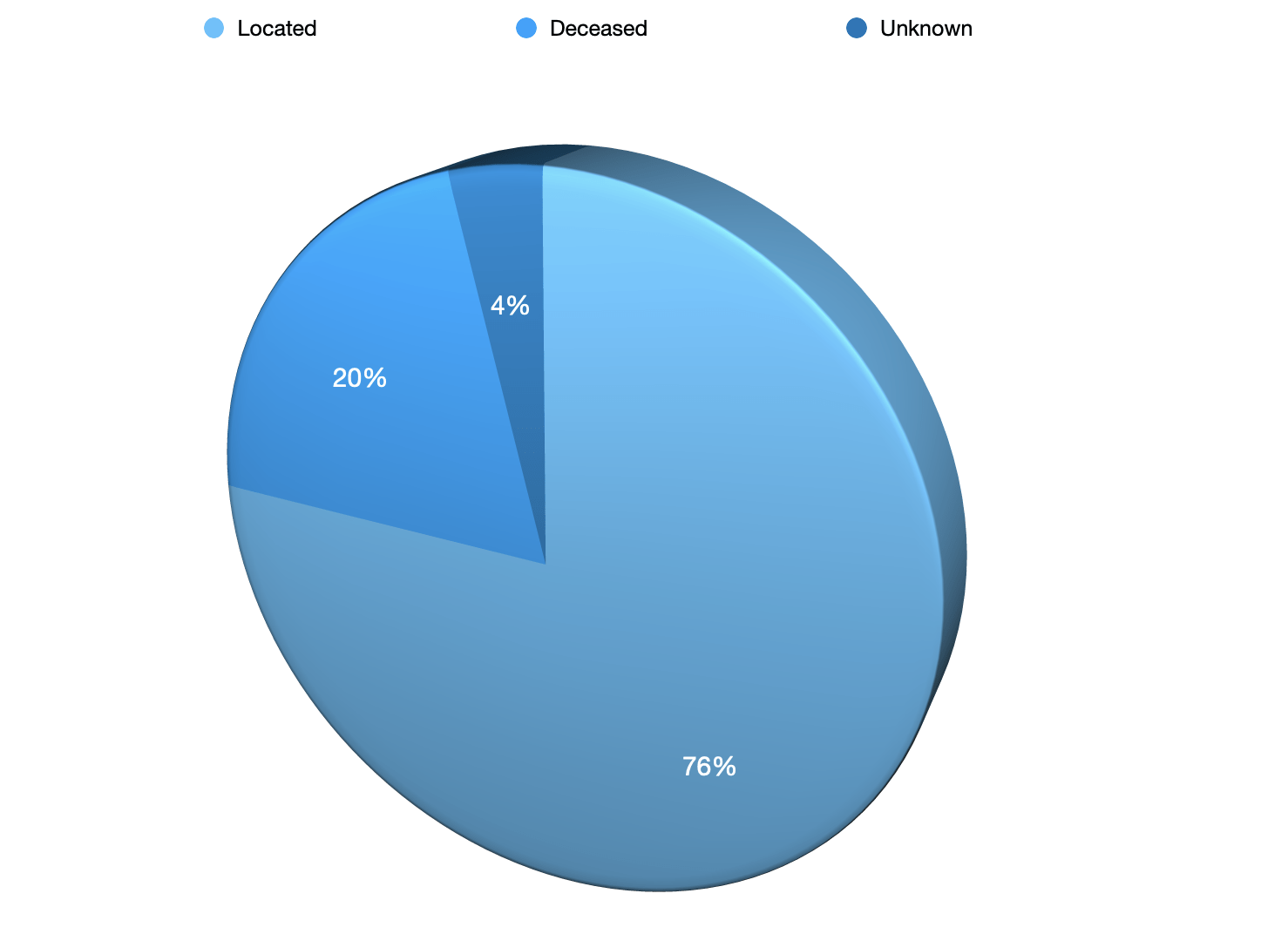
Located 83
Deceased 9
Unknown 6
LTFU Study - File 3
LTFU Global was given the crucial task of locating 271 lost-to-follow-up (LTFU) patients who had previously consented to participate in clinical trials. These patients had become unreachable during the study, posing a major challenge to maintaining data integrity and achieving successful trial results. Reconnecting with them was vital, as their continued involvement was essential for ensuring accurate and comprehensive trial data. Leveraging our expertise in patient tracing and compliance, LTFU Global launched a focused effort using advanced tracing techniques to re-establish contact with the patients.
At LTFU Global, we understand the complexities of tracking LTFU patients, particularly in the context of global clinical trials. To maximise success, we combined innovative technology with personalised outreach. Our approach included verifying contact details, performing vital status checks, and reconnecting with patients who had lost contact with clinical teams. As a result of these efforts, we successfully located 259 patients, significantly enhancing follow-up care and improving the overall accuracy and outcomes of the clinical trials.
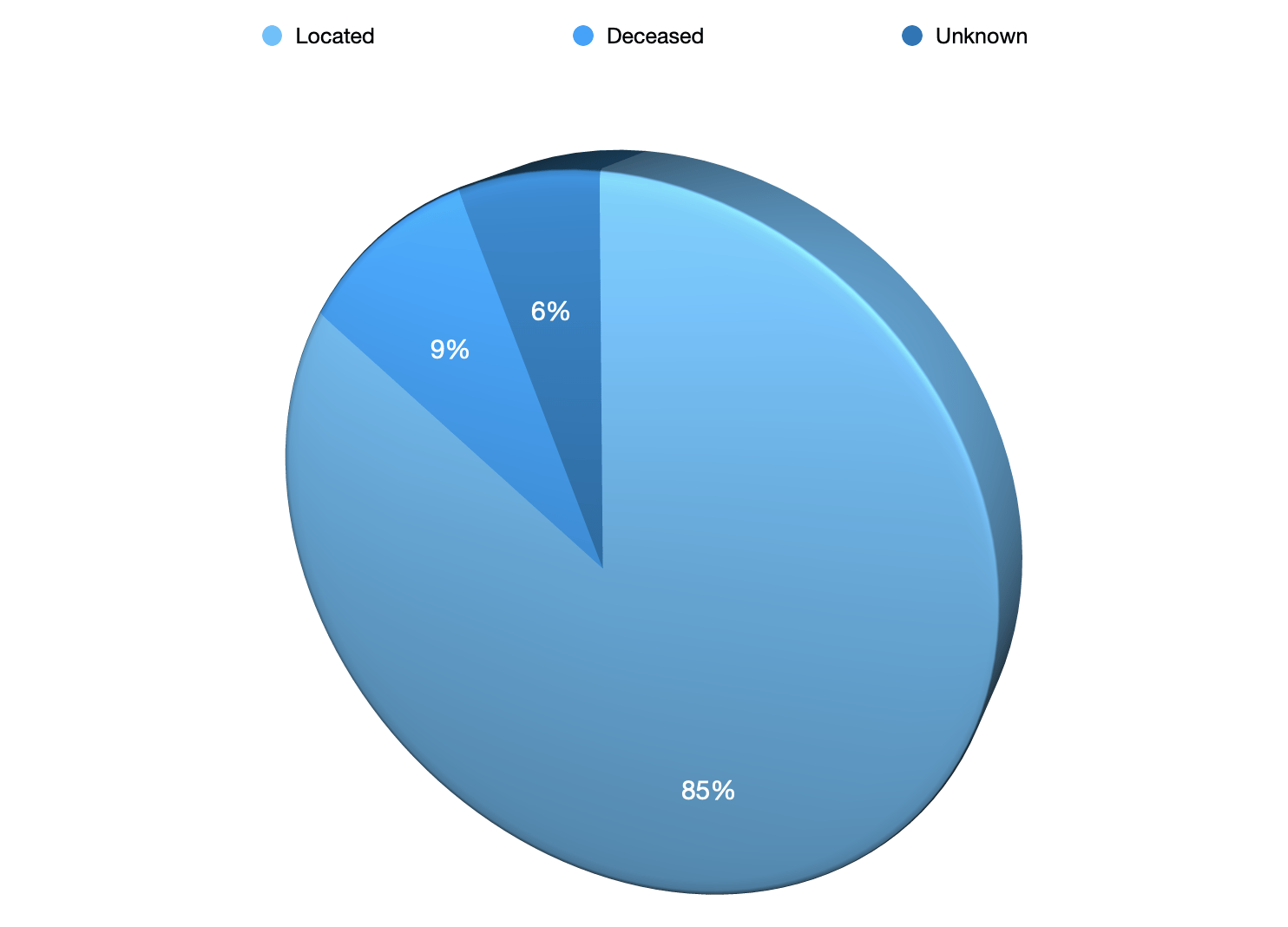
Located 238
Deceased 21
Unknown 12
LTFU Study - File 4
LTFU Global was entrusted with the critical responsibility of finding 482 lost-to-follow-up (LTFU) patients who had previously agreed to participate in clinical trials. These individuals had become uncontactable during the course of the study, creating significant obstacles to preserving data accuracy and ensuring successful trial outcomes. Re-engaging these patients was essential, as their participation was crucial for maintaining reliable and complete trial data. Drawing on our specialized knowledge in patient tracing and regulatory compliance, LTFU Global initiated a dedicated campaign, employing advanced tracking methods to re-establish communication with these patients.
At LTFU Global, we recognise the challenges involved in locating lost-to-follow-up (LTFU) patients, especially within global clinical trials. To ensure the best outcomes, we integrated cutting-edge technology with a tailored outreach strategy. Our method involved validating patient contact information, conducting vital status assessments, and re-engaging patients who had lost communication with clinical teams. Through these targeted efforts, we successfully traced 458 individuals, greatly improving follow-up care and enhancing the accuracy and success of the clinical trial data.
Located 458
Deceased 124
Unknown 24
LTFU Study - File 5
LTFU Global was assigned the important task of locating 204 lost-to-follow-up (LTFU) patients who had previously consented to join clinical trials. These patients had become unreachable during the study, posing major challenges to maintaining data integrity and achieving successful outcomes. Reconnecting with these individuals was vital, as their ongoing involvement was key to ensuring that the trial data remained accurate and comprehensive. Utilising our expertise in patient tracing and adherence to regulatory standards, LTFU Global launched a focused initiative, utilising sophisticated tracking techniques to restore communication with these patients.
At LTFU Global, we understand the difficulties associated with tracking lost-to-follow-up (LTFU) patients, particularly in the context of international clinical trials. To achieve optimal results, we combined state-of-the-art technology with a customised outreach approach. Our strategy included confirming patient contact details, performing vital status evaluations, and reconnecting with individuals who had fallen out of contact with the clinical teams. Thanks to these focused initiatives, we successfully located 193 patients, significantly enhancing follow-up care and improving both the accuracy and effectiveness of the clinical trial data.
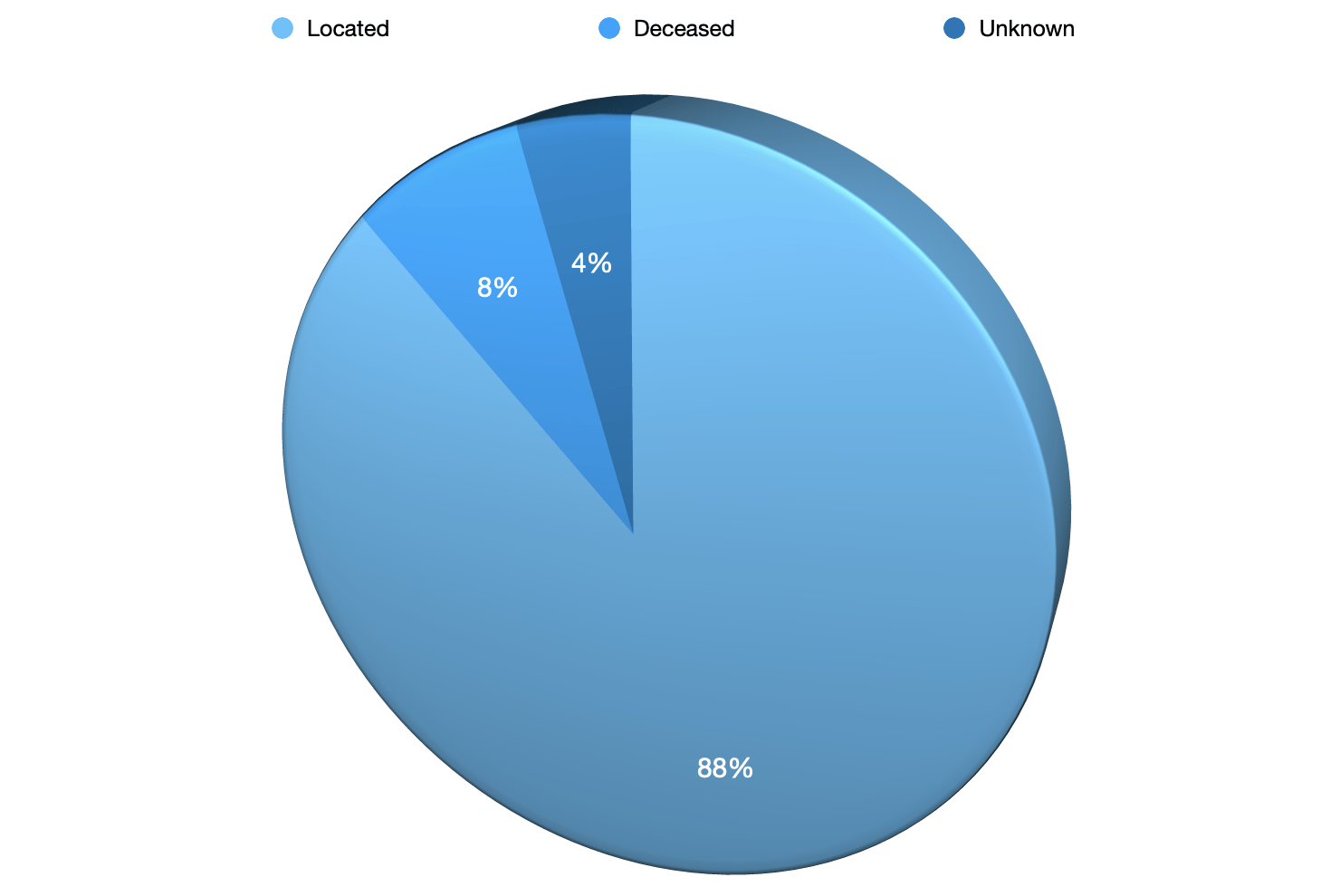
Located 193
Deceased 9
Unknown 11
Summary
LTFU Global established that the amount of patients that were found to be “unknown” varied and were greatly dependant on the following factors:
Type of studies conducted – did the trials relate to serious illnesses or not, this would impact on the amount of deceased patients and also “unknown”
Countries where the searches were conducted – inevitably there will be some countries with lesser resources than others which will then have an impact on establishing the current alive and well status of the patients.
Social and financial demographic of the patients recruited.
How long ago the trials were completed/when did the patients drop off – the more time that passes since the last contact with the patients will have an impact on the alive and well status, change of address and contact details.
Building a team of specialist agents who are also result driven.
How patient data has been transferred – was the data manually transferred/re-typed or written down at any stage, errors do happen and this would effect the “unknown” patients.
The Importance of LTFU Support in Global Clinical Trials
Conducting clinical trials across multiple countries presents unique challenges, making it essential for LTFU support to be adapted to the specific needs of participants in each region. Trials that span across the 165 largest countries worldwide must account for cultural, economic, and infrastructural differences when developing LTFU strategies. For example, methods that successfully engage patients in Canada may not be as effective in regions like India or Brazil, where healthcare systems and societal expectations vary greatly. Tailoring approaches to each region is key to maintaining patient engagement and ensuring the success of global trials.
Why Choose LTFU Global?

Global Expertise
Innovative Solutions

Comprehensive Support


